Bats

Outbreaks of RNA viral disease represent a clear and present danger to human health, societal function, economic and national security, and geopolitical stability. The current COVID-19 pandemic has revealed how unprepared our society was for a novel zoonotic pathogen which yielded devastating results. We were unable to predict, control, and contain the COVID-19 pandemic because we did not heed the warnings presented by related viruses (e.g., SARS-CoV and MERS-CoV) and learn lessons. As we become more aware of zoonotic viral threats, it is important to bear in mind that SARS-CoV-2 has a relatively low case fatality rate of ~2% (Machado et al., 2021). In contrast, emerging infectious diseases, such as hantaviruses, have a relatively high case fatality rate (~30%) (Jonsson et al., 2010). A novel pathogen at 30% mortality infecting 50% of the US population (166.7 million) would result in nearly 50 million deaths in the same timeframe as the COVID-19 pandemic (Machado et al., 2021). A better strategy including surveillance, detection, characterization, and modeling of zoonotic events and epidemic trajectory is needed to predict, respond to, and contain the next pandemic.
Bat reservoirs (in collaboration with Laurel Yohe)
Bats exhibit exceptional tolerance of highly pathogenic viruses (Hendra, Nipah, Ebola, SARS-CoV) without manifesting disease, but the immunological basis of how deadly pathogens can silently reside and circulate within bat populations is poorly understood. Diet is a well-known modulator of priming the microbiome, serving as the ‘training wheels’ of the developing immune system. Different long-term diets should, in theory, shape the gut’s gatekeeping ability to recognize, respond, and expel disease-causing pathogens, but this connection is not well described. The extensive diversity of bats with over 1,100 species occupying the most diverse selection of dietary strategies in any other mammalian order, ranging from numerous forms of insectivory to blood-feeding to fruit specialists, offers the opportunity to explore how evolution has naturally shaped the immune systems of species specialized on different diets. The immense diversity of immunological responses and viral tolerances in bats hint that environmental factors may be at play. Despite variation among species, a series of puzzling trends in ways in which bats respond to viral infections have emerged: a low production of adaptive antibodies, suppressed interferon activity, reduced or absent inflammation, and dampened cytokine storms. To understand how this immune “silencing” may occur and to identify the cellular and nutrient players involved in this decreased signaling, our project investigates the interface of where pathogens can often be most readily detected in bats: the intestine
Rodent reservoirs
Hantaviruses are transmitted via aerosols of rodent excreta to humans, in which the rodent host reservoir has no visible disease (Jonsson et al., 2010). Hantaviruses present a threat due to the high mortality (~30%), rapid evolution through genetic reassortment, high prevalence of rodent-human interaction in domestic settings, rodent migration due to climate change, and documented person to person viral transmission (Martínez et al., 2020). Hantavirus infection in humans leads to two distinct diseases: 1) hemorrhagic fever with renal syndrome (HFRS), and 2) hantavirus cardiopulmonary syndrome (HCPS) (Jonsson et al. 2010). HFRS is primarily caused by Old World hantaviruses [e.g., Hantaan virus (HTNV), Puumala virus (PUUV), Dobrava virus (DOBV), and Seoul virus (SEOV). HCPS is New World disease caused by Sin Nombre Virus (SNV), Andes Orthohantavirus (ANDV), Muleshoe virus (MULEV), Black Creek Canal virus (BCCV), and other hantaviruses (Jonsson et al., 2010). While our models presented in this proposal will be generalizable to more than just hantavirus, the focus of the sampling for model validation will focus on viral lineages that circulate in the USA and neighboring countries. Hantavirus zoonotic events have led to outbreaks in North America. For example, ANDV has evolved new modes of transmission, including three confirmed person-person transmission events, and >1,200 cases in Argentina since 1995 (Martínez et al., 2020). In the Old World case counts are much higher. Annual cases of HFRS are >60,000-100,000 from Old World hantavirus of which 90% are reported in China, Russia, and Korea with a mortality 0.43% to 15% (Li et al., 2019; Romero & Anjum, 2021). Currently, no Old World hantavirus has been shown to transmit human to human.
Hantavirus has also expanded its geographic range across North America since the SNV 1993 outbreak. Historically 90% of human hantavirus cases occurred west of the Mississippi river (Fig 1) (CDC, 2021). Recently, human hantavirus cases are expanding east of the Mississippi, and northward (Berl et al. 2018). These changes may be linked to hantavirus host range expansion, and climate change (Chen et al., 2019). For example, Michigan had its first documented case of hantavirus (i.e., SNV) in mid-2021 (Shamus, 2021). In Canada, from 1989 to 2020, 143 positive HCPS cases have been confirmed to be caused by SNV-infected the deer mouse (Peromyscus maniculatus) (Warner et al., 2020). Furthermore, the eastern expansion of hantaviruses in the USA will continue to be exacerbated by climate change over the next 10-50 years (Klempa, 2009). A changing climate will push mammalian host range expansion due to resource limitation, altering weather patterns, severe weather changes (e.g., droughts and floods), habitat fragmentation, and habitat loss. Moreover, activities driven by human population expansion and subsequent habitat conversion (e.g., housing developments) create more opportunities for human-rodent-virus interactions (Klempa, 2009; Pitts et al., 2013). The classic example linking a hantavirus outbreak to climate is the 1993 Four Corners outbreak in the southwestern USA. In this case, the El Niño-Southern oscillation event increased rainfall, leading to an increase in the population size of the SNV host, P. maniculatus. These events were followed by increased rodent-human interaction in buildings and subsequent zoonotic transmission (Klempa, 2009). Landsat data substantiated these findings (Glass et al., 2006).
Given a diversity of hantavirus lineages present in North America (SNV, MULEV, BCCV), a major concern is that genetic reassortment will result in highly virulent virus that transmits person-person resulting in a major outbreak. Genetic reassortment is a process when two or more viral lineages infect the same host cell, then due to having their genome on multiple segments reassort and shuffle their segments into new viral genotypes. Pandemic influenza H1N1 2009 resulted from a reassortment event between three different H1N1 lineages (Trifonov et al., 2009). Many hantaviruses have evidence of genome reassortment including SNV, PUUV, DOBV, HTNV, and SEOV (Klempa, 2018). The Camp Ripley Virus (RPLV) has been shown to undergo genetic reassortment under natural conditions and occurs east of the Mississippi River (Liphardt et al., 2020). In vitro experiments have shown that ANDV and SNV, SNV and BCCV, and SNV of two distinct lineages can reassort resulting in hybrid progeny viruses (Klempa, 2018).
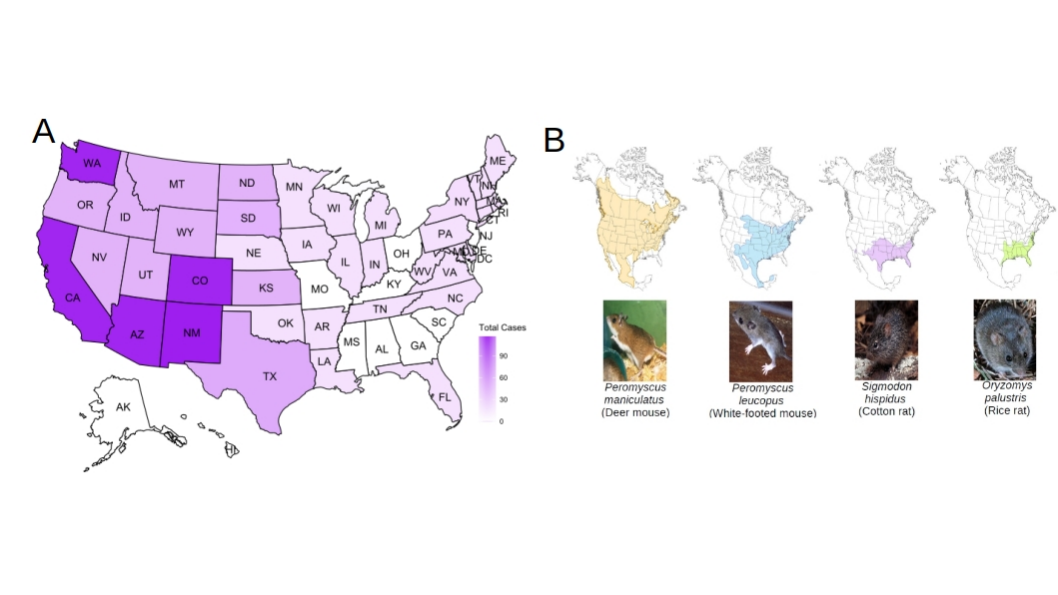
Fig 1.Total hantavirus cases by state and selected rodent host distribution. A) Map of all human cases of hantavirus infections from 1993-2019. Five cases had presumed exposure outside the USA. B) Selected hantavirus mammalian host species across North America associated with outbreaks. This is not a complete list of known carriers of hantavirus (e.g., voles, shrews, bats) (Sabino-Santos et al., 2018). Source: CDC 2021.
Known rodent hosts of hantaviruses that have led to disease outbreaks in humans include Apodemus agrarius (striped field mouse; carries HTNV), Apodemus flavicollis (yellow-necked mouse; carries DOBV), Myodes glareolus (bank vole; carries PUUV), Oligoryzomys longicaudatus (long-tailed pygmy rice rat; carries ANDV), Oryzomys palustris (marsh rice rat; carries Bayou Virus [BAYV]), Peromyscus leucopus (white-footed mouse; carries New York Virus [NYV] and Monongahela Virus [MGLV]), Rattus norvegicus (Norway rat; carries SEOV), and Sigmodon hispidus (hispid cotton rat; carries MULEV and BCCV) (Holmes & Zhang, 2015). In North America, rodents of special concern that are known as asymptomatic carriers and are chronically infected with hantaviruses which have caused known outbreaks in humans include P. maniculatus, P. leucopus, O. palustris, and S. hispidus (Fig 1). P. maniculatus has the largest known distribution of these species, extending from the Yukon Territory in Canada south across the entire Western USA and east to New York state (Fig 1). The primary hosts in the USA linked to hantavirus outside of known SNV distribution (east of the Mississippi River) are S. hispidus (Florida), P. leucopus (New York), and P. maniculatus (Pennsylvania/West Virginia) (Knust and Rollin, 2013). P. leucopus is especially problematic as it carries MGLV and NYV, which are both known to cause human outbreaks. P. leucopus also carries Blue River (BRV), which has unknown human pathology (Hjelle & Torres-Pére, 2010). Genomic reassortment of hantaviruses involving P. leucopus is likely due to range overlap with multiple other rodents within the eastern USA, as well as its competence for at least three distinct hantaviruses.
We currently lack complete reference genome sequences for many lineages of Hantavirus. GenBank includes over 11,000 Orthohantavirus nucleotide sequences that we can leverage to identify viruses that we collect from known hantavirus positive samples archived in the Museum of Texas Tech University (TTU) genetic resources collections. However, only 33 complete reference genomes representing a specific clade of hantavirus (e.g., SNV or SEOV) are present within GenBank. Viral genomes are needed for improved diagnostics and treatment as well as analyses of mutation, reassortment, recombination, and phylogeny to understand the spread, ecology and evolution of hantaviruses.