Microbialities
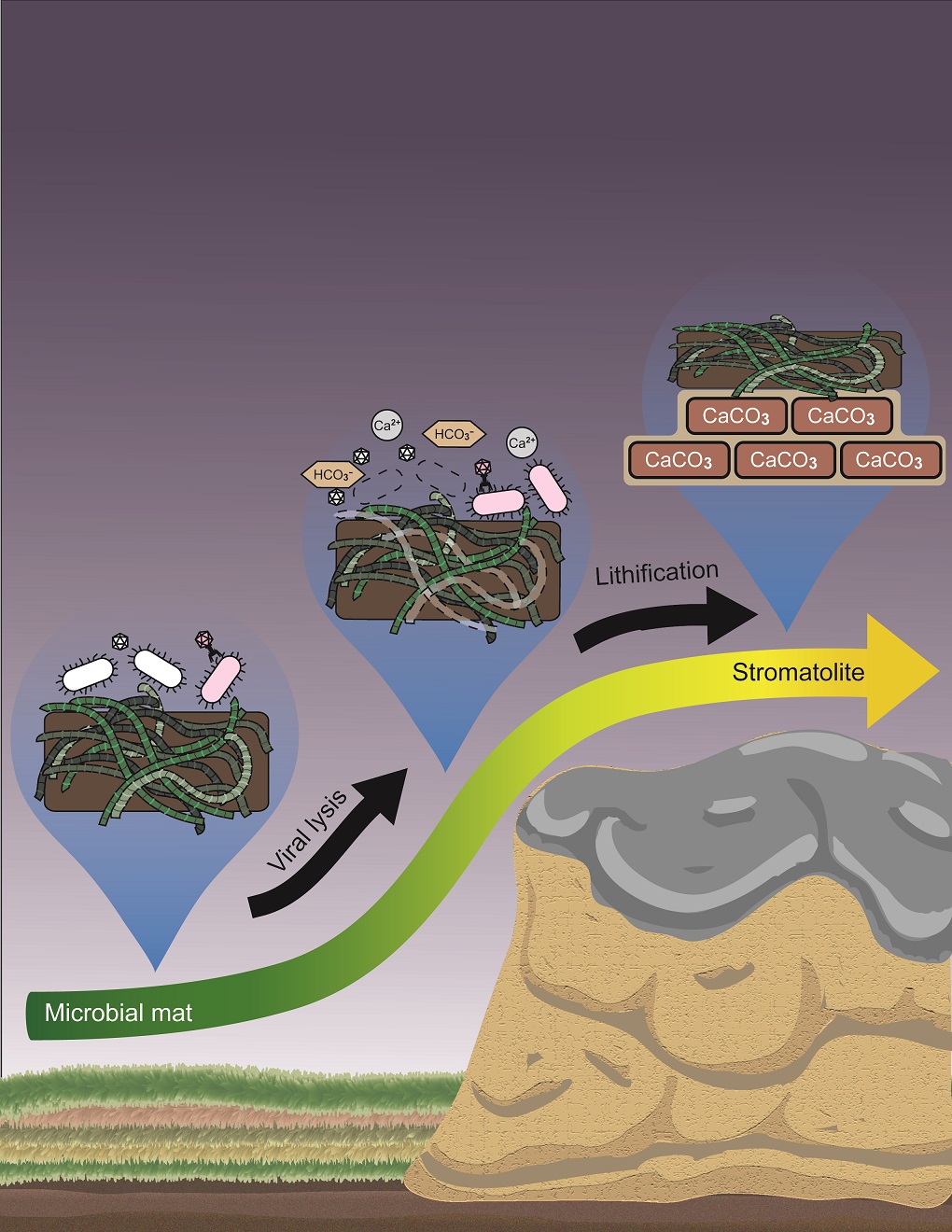
Microbialites represent the oldest evidence for life on the planet. For around 3.7 billion years, 85% of geological time, microbialites have thrived and colonized the globe within ecosystems representing soil, marine and fresh water, and geothermal hot springs. Modern versions of microbialites still exist today in the form of a specialized group of cyanobacteria-dominated microbial mats that lithify carbonate-rich structures, which allows for geological preservation. They provide geological evidence of them, in a way, ancient time capsules. Microbialite ecosystems transform carbon dioxide into mineral carbonates, trapping those gases reversibly for up to millions of years. Microbialites are vulnerable ecosystems in this era of climate change and ocean acidification, both of which threaten their existence. Meanwhile, their primary function is to trap light and carbon through photosynthetic and heterotrophic processes. This results in biomass production that provides a kind of oasis within oligotrophic ecosystems. Less is known how these networks of organisms interact to sustain themselves and to actively precipitate carbonates. For example, if one bacterial group overgrows and produces too many organic acids, the change in pH would cause microbialites to dissolve, and would trigger a collapse of the ecosystem. Microbialites have thrived and have occupied a variety of habitats across the globe. However, all exist in a near-perfect balance of phototroph-heterotroph abundance. This balance keeps grazing pressure to a minimum and does not allow viruses to completely wipe out the principal members of a community (for example, the cyanobacterial mat builders).
Microbialites exist at the interface of chemistry and biology. The chemistry selects particular microbes, then the microbes as a byproduct (or active process) of metabolism precipitate carbonates. Microbialites represent the simplest model of a complex microbial ecosystem in which phototrophic cyanobacteria and heterotrophic bacteria interact. Since they are vulnerable ecosystems (for example, vulnerable to ocean acidification) and since microbialites are sensors of environmental change, I would like to use them as a model for how climate will impact more complex microbial communities. They represent a unique opportunity that is simple, easy to apply, and easy to model.
My previous work on microbialites includes metagenomic examination of both freshwater microbialites of Pavilion Lake (White III et al., 2016, Frontiers) and Clinton Creek (White III et al., 2015, Frontiers) in Canada and marine microbialites of Shark Bay Australia (Rendy, White III et al., 2016; Wong, White III et al., 2018, ISMEJ). We have published with collaborators over >100 Metagenome-assembled genomes (MAGs) affiliated to microbial dark matter (rare yet to be cultivated phyla), spanning 42 phyla (Wong, White III et al., 2018 ISMEJ; Wong, White III et al., 2020, Microbiome). These phyla were capable of fermenting and degrading organic carbon, suggesting a role in recycling organic carbon, dissimilatory sulfate reduction, and schizorhodopsins for capturing light energy. We also found that diversity-generating retroelements were prominent in DPANN archaea that likely facilitate the adaptation to a dynamic, host-dependent lifestyle. These rare phyla included Parcubacteria, Asgard archaea, DPANN archaea, Zixibacterial order GN15, and Bathyarchaeota. The Wood–Ljungdahl pathway is an ancient pathway for carbon assimilation and was over-represented in Shark Bay microbialites. Viral defense mechanisms such as CRISPR-Cas, BREX, and DISARM systems were discovered amongst the MAGs, suggesting rampant bacterial phage infections and interactions. A follow-up study published this year was the first description of viruses in Shark bay and only the second paper ever published on viruses in microbialites (White III et al., 2018, Frontiers). My study on the Shark Bay viromes details the viral community diversity, host-viral defense mechanisms, and compares the viromes of other marine and freshwater microbialites.